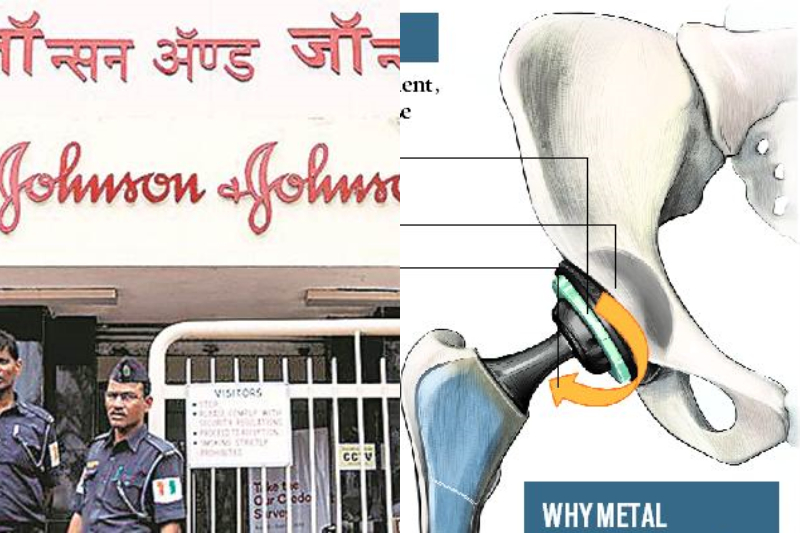
The Health Ministry has accepted the recommendation of experts panel and formed the Central Committee. They also have directed all the states to form a committee at the state level to counter the number of patients is affected by the Johnson & Johnson faulty hip implant.
In the year, 2017, the Health Ministry had formed a committee of experts including Dr. Arun Agrawal, dean of Maulana Azad Medical College. The panel suggested that, each patient who has been affected by the Johnson & Johnson faulty hip implant to be paid Rs 20 lakh.
“State-level committees, instead of regional committees, should be formed to enable hassle-free access to the patients as they can easily approach the state level committee in their respective states. The patients can also be given preference to approach either of the two committees (Central or State),” said a notification dated August 30, 2018.
The committee will be formed comprising, one radiologist from the government medical college and a representative from the zonal office of drug regulator. All the States have been asked to take out advertisements in newspapers so that affected patients can approach the committees.
Currently, there are no specific legal provisions to provide compensation to patients in such cases. The government swung into action when a newspaper exposed the case and highlighted concerns over the metal-on-metal acetabular surface replacement (ASR) hip implant.
Also read: Gauri Lankesh murder case: Parashuram Waghmare shot scribe, claims SIT
Who can apply for the Compensation?
All patients who were implanted with ASR will be eligible to apply to the state-level and central expert committees for compensation. According to the health ministry, the first surgery of ASR must have been performed in India on or after 2006 and the patients must have been fitted with ASR within 10 years from the date of first surgery. Patients who have been implanted with ASR between 10-15 years from the date of first surgery, but are symptomatic, will also be eligible to apply.
“The central expert committee with the assistance of the state-level committee will arrive at the exact amount of the compensation as admissible under appropriate law, which will be communicated to Central Drugs Standard Control Organisation (CDSCO), which will pass an order for a grant for compensation,” the letter said.
Earlier, the committee had held the ASR hip implants manufactured by DePuy International Ltd to be faulty. “The committee is of the considered view that the revision surgeries were necessitated due to the faulty ASR as well as negligence of the firm in approaching the patients and therefore it is the responsibility of the firm to compensate all affected patients,” the committee report said.
J&J has been in the dock for adopting double standards in paying compensation in India contrary to a hefty compensation of $2.5 billion that it agreed to pay to around 8,000 US citizens who had sued the company following faulty hip implants.
According to the report, “A reconstruction of J&J’s handling of hips implants, based on interviews and internal company documents revealed that it was “evasive” in providing the information.”
The DePuy spokesperson reiterated that the company’s actions concerning the product were “appropriate” and “responsible”. “It is also important to note that a voluntary recall doesn’t imply that the product is faulty. Nor does it imply that every patient who has received an ASR hip implant will necessarily have to undergo revision surgery. Indeed, ASR continues to function well for many patients in India and around the world,” the spokesperson said.
Also read: Tamil Nadu Gutkha Scam: CBI raids at Health Minister and DGP residence