- Sponsored -
India’s First Coronavirus Vaccine: Covaxin’s Human Trials To Begin Today
As Per The Latest Reports, India First Coronavirus Vaccine-Covaxin's Human Trials Begins From Today
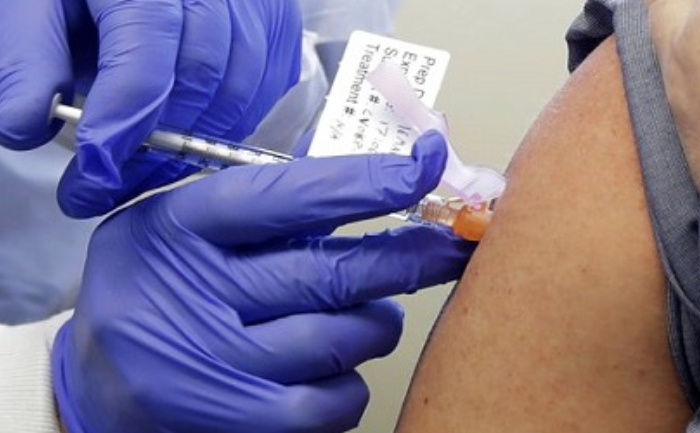
The pandemic Coronavirus has been spreading like the wildfire all across globe. With the increasing number of COVID-19 cases, scientists have been racing to invent the vaccines to treat people. India’s first indigenous Coronavirus vaccine, Covaxin gets a nod for its first human trials today.
- Sponsored -
As per the latest reports, the human trials will begin from today. AIIMS-Delhi is among the 12 sites selected by the Indian Council for Medical Research (ICMR) for conducting the phase I and II human trials of Covaxin vaccine. The DCGI(Drug Controller General Of India) has permitted two vaccines, one which is developed by the Bharat Biotech International Limited in collaboration with the ICMR and another one by Zydas Cadila Healthcare Ltd to go in for phase I and II human clinical trials.
Apparently, in the first phase the vaccine would be tested on 375 volunteers and a maximum of 100 of them would be from AIIMS. Healthy volunteers who doesn’t have any comorbid conditions and volunteers without a history of COVID-19, aged more than 18 years and less than 55 years, would be eligible to participate in the controlled clinical trials of Covaxin.
Approximately 1,000 human volunteers would be participating in the exercise for each of the two indigenously developed vaccine candidates, as per the reports.
For more such updates, stay hooked on to The Live Mirror.
Also Read: COVID-19: India Reports 38,902 New Coronavirus Cases, Highest In 24 Hours
- Sponsored -
